Isoleucine
From Wikipedia, the free encyclopedia
| L-Isoleucine | |
|---|---|
|
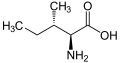
Isoleucine
|
|
|
other names
2-Amino-3-methylpentanoic acid
|
|
| Identifiers | |
| CAS number | 73-32-5 |
| PubChem | 791 |
| SMILES |
CC[C@H](C)[C@H](N)C(O)=O
|
| Properties | |
| Molecular formula | C6H13NO2 |
| Molar mass | 131.17 g mol−1 |
| Supplementary data page | |
| Structure and properties |
n, εr, etc. |
| Thermodynamic data |
Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
Isoleucine (abbreviated as Ile or I)[1] is an α-amino acid with the chemical formula HO2CCH(NH2)CH(CH3)CH2CH3. It is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested. Its codons are AUU, AUC and AUA.
With a hydrocarbon side chain, isoleucine is classified as a hydrophobic amino acid. Together with threonine, isoleucine is one of two common amino acids that have a chiral side chain. Four stereoisomers of isoleucine are possible, including two possible diastereomers of L-isoleucine. However, isoleucine present in nature exists in one enantiomeric form, (2S,3S)-2-amino-3-methylpentanoic acid.
Contents |
[edit] Biosynthesis
As an essential amino acid, isoleucine is not synthesized in animals, hence it must be ingested, usually as a component of proteins. In plants and microorganisms, it is synthesized via several steps, starting from pyruvic acid and alpha-ketoglutarate. Enzymes involved in this biosynthesis include:[2]
- Acetolactate synthase (also known as acetohydroxy acid synthase)
- Acetohydroxy acid isomeroreductase
- Dihydroxyacid dehydratase
- Valine aminotransferase
[edit] Catabolism
Isoleucine is both a glucogenic and a ketogenic amino acid. After transamination with alpha-ketoglutarate the carbon skeleton can be converted into either Succinyl CoA, and fed into the TCA cycle for oxidation or conversion into oxaloacetate for gluconeogenesis (hence glucogenic). It can also be converted into Acetyl CoA and fed into the TCA cycle by condensing with oxaloacetate to form citrate. In mammals Acetyl CoA cannot be converted back to carbohydrate but can be used in the synthesis of ketone bodies or fatty acids, hence ketogenic.
[edit] Isomers of isoleucine
| Forms of Isoleucine | |||||||
|---|---|---|---|---|---|---|---|
| Common name: | isoleucine | D-isoleucine | L-isoleucine | DL-isoleucine | allo-D-isoleucine | allo-L-isoleucine | allo-DL-isoleucine |
| Synonyms: | (R)-Isoleucine | L(+)-Isoleucine | (R*,R*)-isoleucine | alloisoleucine | |||
| PubChem: | CID 791 | CID 94206 | CID 6306 | CID 76551 | |||
| EINECS number: | |||||||
| CAS number: | 443-79-8 | 319-78-8 | 73-32-5 | 1509-35-9 | 1509-34-8 | 3107-04-8 | |
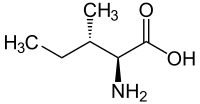  |
| L-isoleucine (2S,3S) and D-isoleucine (2R,3R) |
  |
| L-allo-isoleucine (2S,3R) and D-allo-isoleucine (2R,3S) |
[edit] Synthesis
Isoleucine can be synthesized in a multistep procedure starting from 2-bromobutane and diethylmalonate.[3] Synthetic isoleucine was originally reported in 1905.[4]
[edit] References
- ^ IUPAC-IUBMB Joint Commission on Biochemical Nomenclature. "Nomenclature and Symbolism for Amino Acids and Peptides". Recommendations on Organic & Biochemical Nomenclature, Symbols & Terminology etc. http://www.chem.qmul.ac.uk/iupac/AminoAcid/. Retrieved 2007-05-17.
- ^ Nelson, D. L.; Cox, M. M. "Lehninger, Principles of Biochemistry" 3rd Ed. Worth Publishing: New York, 2000. ISBN 1-57259-153-6.
- ^ "dl-Isoleucine", Org. Synth., 1955, http://www.orgsyn.org/orgsyn/orgsyn/prepContent.asp?prep=cv3p0495; Coll. Vol. 3: 495
- ^ Bouveault and Locquin, Compt. rend., 141, 115 (1905).
[edit] External links
|
||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||